Genotoxicity Database
OASIS genotoxicity database was created and is maintained by LMC.
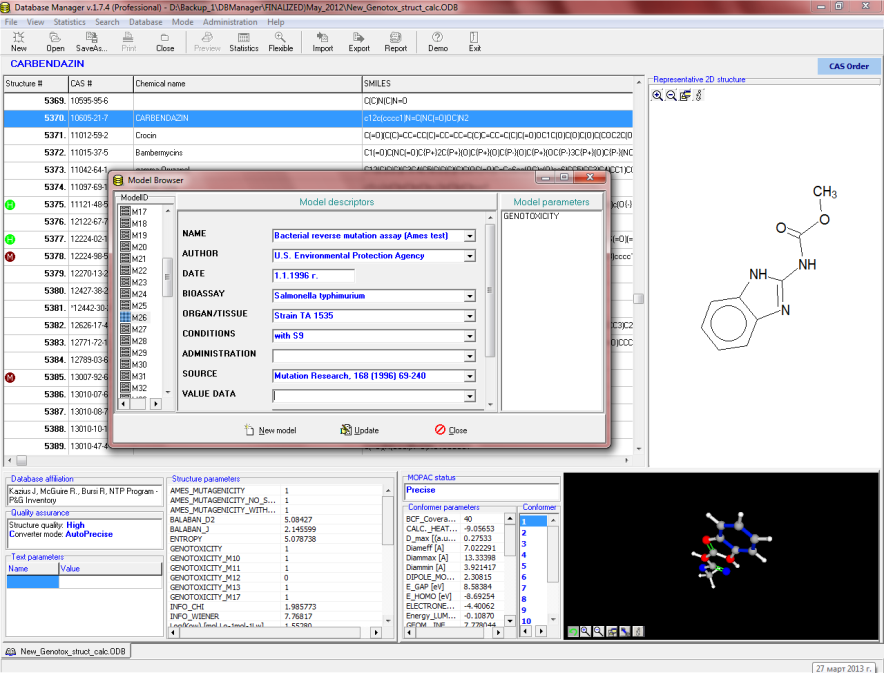
Chemical Identification
Chemicals are defined with CAS, Name, SMILES. The database can be managed by using OASIS Database Manager. This platform has an intelligent search option which allows extraction of chemical subsets for further analysis or model developments.
Content
OASIS genotoxicity database contains a large collection ofexperimental data (7404 entries) on chemicals for which information about different in vitro genotoxic effects (e.g., Ames test, chromosomal aberrations, mutagenicity in the mouse lymphoma assay) are available [1-4]. The experimentally observed genotoxicity data could be summarized as follows:
- Bacterial mutagenesis (Strains: TA 97, 98, 100, 1535, 1537),
- Without metabolic activation (No S9) - 1860 chemicals,
- With metabolic activation (With S9) - 1775 chemicals,
- Without metabolic activation (No S9) - 515 chemicals,
- With metabolic activation (With S9) - 162 chemicals,
- In vitro chromosomal aberration (Chinese hamster or lung/ovary cells),
- Mouse lymphoma assay (not specified metabolic activation) - 300 chemicals.
The genotoxicity database database has been used to derive mechanistic structure activity-based in vitro models taking into account metabolic activation and detoxification of chemicals.
References
1. K. Mortelmans, S. Haworth, T. Lawlor, W. Speck, B. Tainer, E.Zeiger. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals.Environ Mutagen.1986;8 Suppl 7, 1-119.
2. T. Sofuni,. Data Book of Chromosomal Aberration Test In Vitro, Revised Edition. Life-Science Information Center; (1998) Tokyo, Japan.
3. D. Kirkland , M. Aardema , L. Henderson , L. Müller. Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens I. Sensitivity, specificity and relative predictivity. Mutat Res. (2005), 584 (1-2), pp.1-256.
4. L. Kier, D. Brusick, A. Auletta, E.Von Halle, M. Brown, V. Simmon, V. Dunkel, J. McCann, K. Mortelmans, M. Prival, T.K. Rao, V. Ray. The Salmonella typhimurium/mammalian microsomal assay: A report of the U.S.Environmental Protection Agency Gene-Tox Program. Mutation Research, 168 (1996), pp. 69-240.