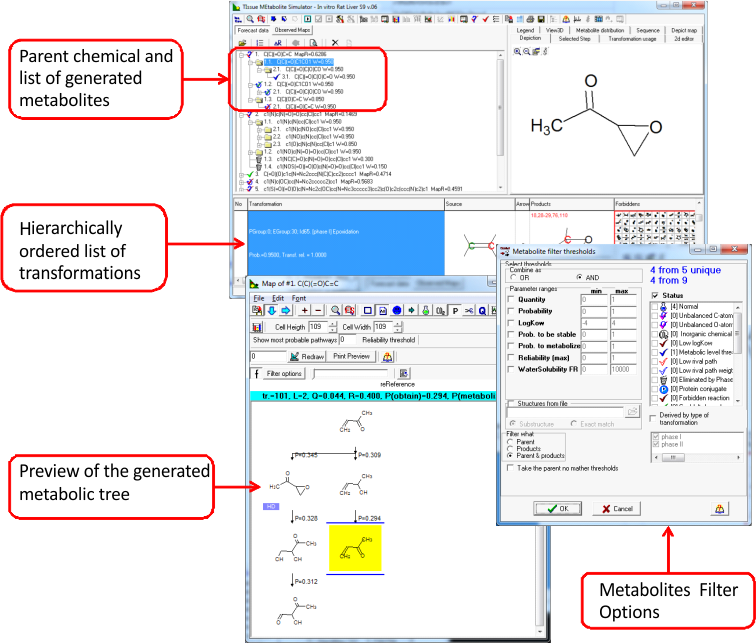
In vitro Rat S9 Metabolism
Endpoint
The in vitro rodent microsomal/S9 metabolic simulator (transformation table) reproduces and predicts the metabolic pathways of xenobiotic chemicals for in vitro experimental systems such as rodent (mostly rat) liver microsomes and S9 fraction.
Data
The metabolism training set contains experimentally observed (documented) in vitro metabolic pathways for 261 parent chemicals of a wide structural diversity, and 1070 observed metabolites compiled into a searchable electronic database. Published data on the metabolism of these chemicals in rodent (mostly rat) liver microsomes and S9 fraction, collected mainly from research publications in scientific journals and, also, from some websites were extracted and introduced into an electronic database [1].
Model
The simulation of metabolism is focused on the correct reproduction of experimentally observed metabolites [2, 3]. The current in vitro rat liver metabolic simulator represents electronically designed set of 509 structurally generalized, hierarchically arranged biotransformation reactions. These molecular transformations are characteristic for the metabolism in the presence of in vitro experimental systems such as rodent (mostly rat) liver microsomes and S9 fraction. Each transformation in the simulator consists of source and product structural fragments, and inhibiting "masks". A probability of occurrence is ascribed to each transformation, which determines its hierarchy in the transformation list. Thus the modeling is based on the set of principal molecular transformations, and the in vitro "logic" of the commonly observed metabolism with the corresponding experimental systems.
The following types of molecular transformations are included into in vitro simulator:
- 25 - 30 abiotic (non-enzymatic) and, also, a few enzyme-controlled reactions believed to occur at a very high rate as compared to the duration of the tests. The highest priority (probability of occurrence) is assigned to these reactions. This subset of reactions includes also transformations of highly-reactive functional groups and intermediates, such as tautomerizations, arene epoxide rearrangements to phenols, etc. which occur spontaneously.
- 450 - 470 enzymatic phase I (mostly CYP450-catalyzed) transformations such as aliphatic C-oxidation, aromatic C-hydroxylation, oxidative N- and O-dealkylation, epoxidation, ester and amide hydrolysis, carbonyl group reduction, nitro and azo group reduction, N-hydroxylation, etc.
- 15 - 20 enzymatic phase II transformations, such as glucuronidation, sulfation, glutathione conjugation, N-acetylation, etc. Significantly lower priorities are assigned to these transformations as compared to phase I ones in accordance with the observed in vitro metabolism pattern.
Domain
The derivation of the structural domain of simulator is based on atom-centered fragments.
Statistics
Average sensitivity (probability that the metabolite is predicted, given that the metabolite is truly observed) is 80 %.
Average predictability (probability that the metabolite is truly observed, given that the metabolite is predicted) is 36 %.
References
1. Kolanczyk R. C,
P. Schmieder, W. J. Jones, O. G. Mekenyan, A. Chapkanov, S.
Temelkov, S. Kotov, M. Velikova, V. Kamenska, K. Vasilev, G.
D.Veith, MetaPath: An Electronic Knowledge Base for Collating,
Exchanging and Analyzing Case Studies of Xenobiotic Metabolism,
Regul. Toxicol. Pharmacol. 63(1) (2012), 84 - 96.
2. Mekenyan, O., S. Dimitrov, T. Pavlov, G.
Dimitrova, M. Todorov, P. Petkov, S. Kotov, Simulation of Chemical
Metabolism for Fate and Hazard Assessment. V. Mammalian Hazard
Assessment, SAR QSAR Environ Res. 23(5-6) (2012), 553 -
606.
3. Serafimova, R., M. Todorov, T. Pavlov, S.
Kotov, E. Jacob, A. Aptula, O. Mekenyan, Identification of the
Structural Requirements for Mutagenicity by Incorporating Molecular
Flexibility and Metabolic Activation of Chemicals. II. General Ames
Mutagenicity Model, Chem. Res. Toxicol. 20 (2007), 662 -
676.