Criteria for assessing the reliability of the predictions: II. CATALOGIC BOD and BCF base-line models
Computational Toxicology Volume 17 (2021) 100154
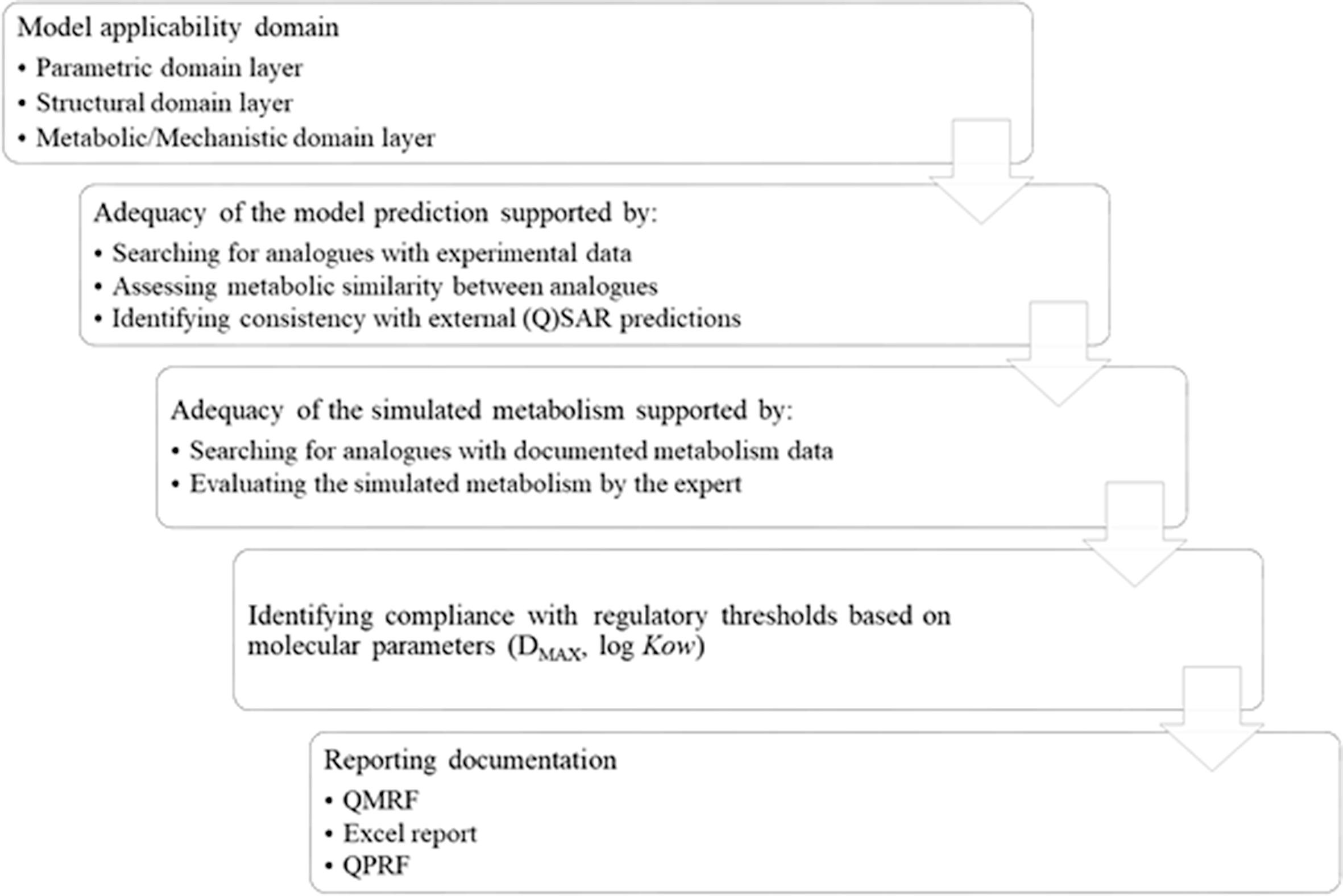
The OASIS CATALOGIC software is distinctive in that it requires the simulation of microbial metabolism for prediction of biodegradation and fish liver metabolism for prediction of bioaccumulation. The reliability of these structure-activity predictions typically involves invoking OECD principles and ECHA practical guidance. Questions remain, especially whether some predictions for chemicals belonging to less than 100% of applicability domain (AD) still may be considered as reliable and what's the tolerated threshold for belonging to a model's AD. Here the reliability of the predictions associated with biodegradation and bioaccumulation endpoints obtained from OASIS CATALOGIC models are examined to clarify criteria for assessing the reliability of the predictions. In the end, the reliability of any prediction is based in large part on: 1) establishing a transparent stream of relevant information, 2) providing and documenting relevant in silico and experimental data, and 3) demonstrating metabolic similarity between chemicals. Moreover, when the metabolic transformation is fundamental, it is critical to establishing the adequacy of the simulated transformations as this is indispensable evidence for establishing the reliability of a prediction.