Criteria for assessing the reliability of toxicity predictions: I. TIMES Ames mutagenicity model
Computational Toxicology Volume 17 (2021) 100143
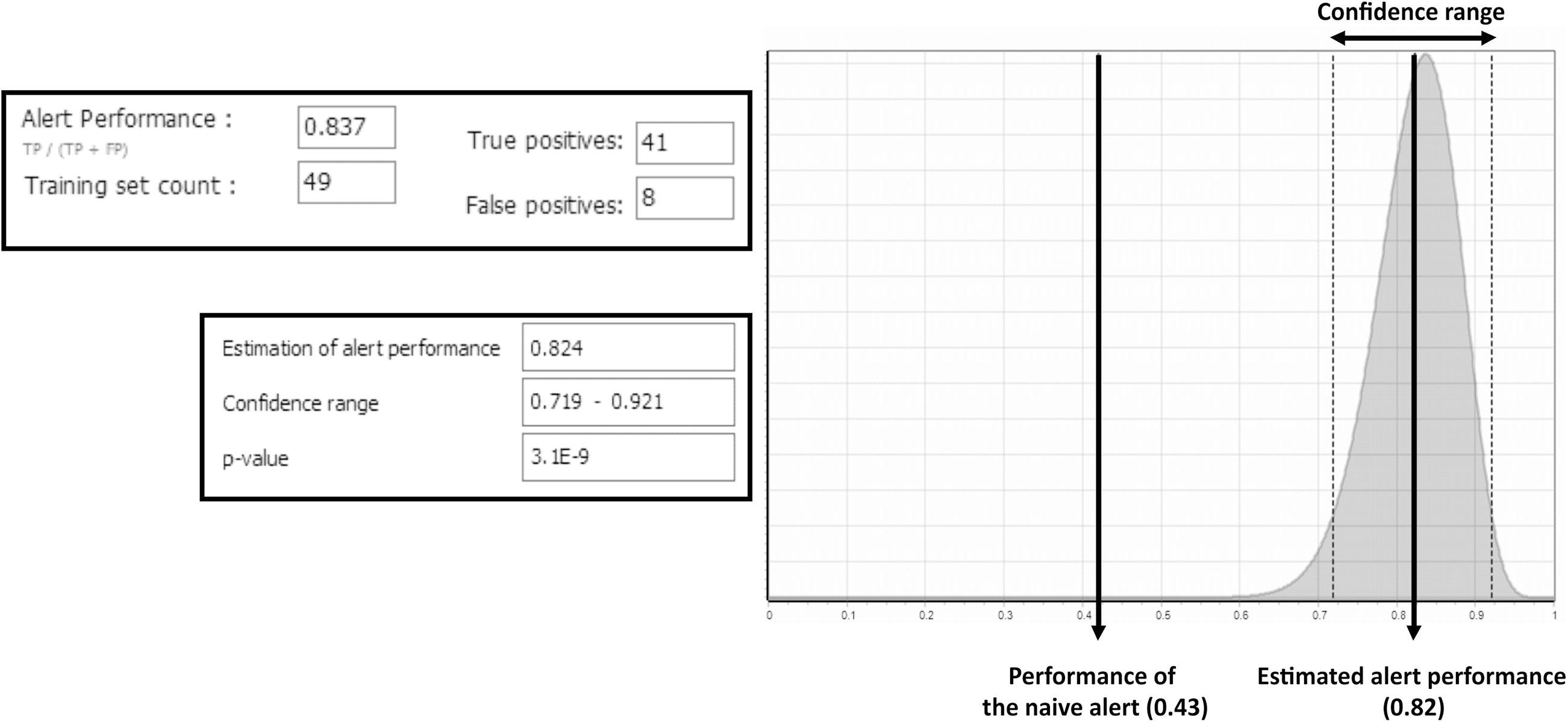
The strain-dependent bacterial reverse mutation assay (Ames test) is typically part of regulatory batteries of methods used for in vitro evaluation of substances eliciting mutagenicity via short-chain-length DNA damage. This study was undertaken in an effort to make the Ames endpoint more amenable to category formation to support assessments of mutagenesis via non-test methods. The plethora of Ames data has allowed for the establishment of well-document mechanistic-based structural alerts and thereby, DNA-binding profiles. These alerts/profiles are linked to positive and in some cases negative response categories for chemicals. These mechanistic categories form a strong basis for addressing the reliability of predictions as the results obtained from such models are likely to be transparent and mechanistically justifiable. Such mechanistic transparency establishes and rationalizes the structure-property causality principle and thus, adds important weight-of-evidence to the prediction. In the present study a set of criteria for evaluating reliability of the TIMES Ames with S9 (ver. 17.17) predictions are presented and demonstrated. These criteria are consistent with previous considerations; however, they stress mechanistic considerations based on metabolism, especially metabolic activation of non-reactive parent chemicals.