Estimating the reliability of simulated metabolism using documented data and theoretical knowledge. QSAR application
Computational toxicology (2022)
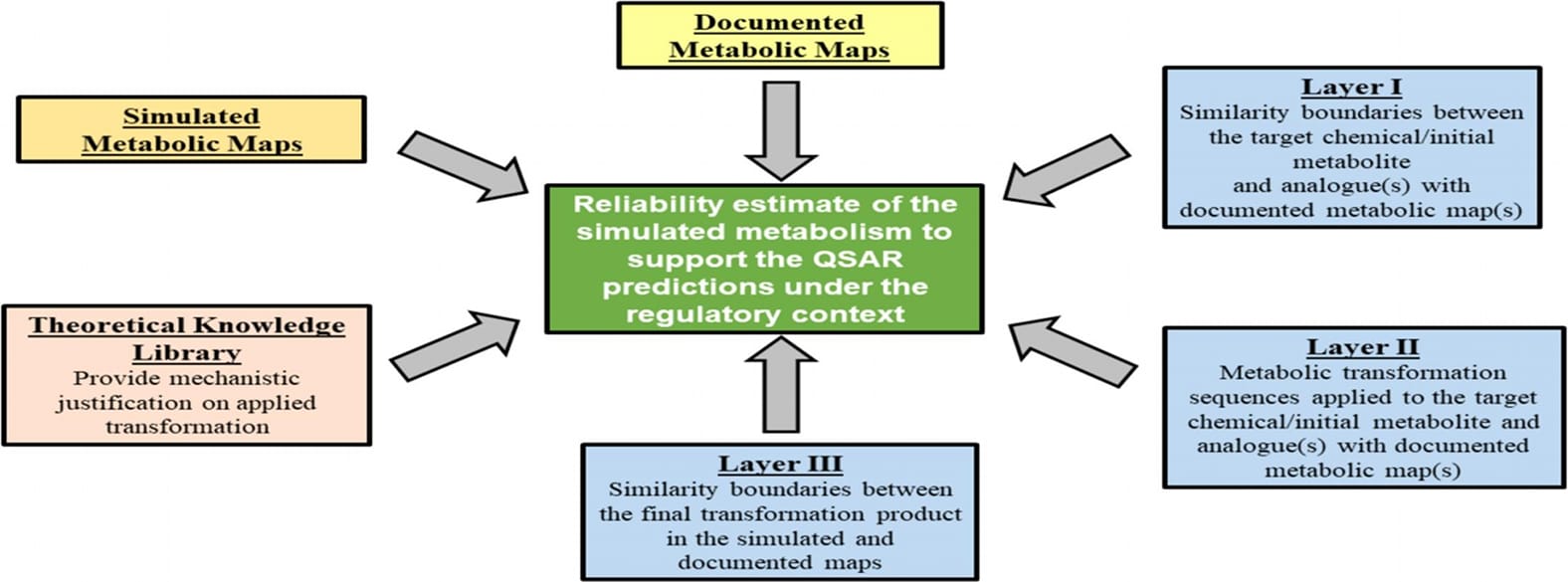
Establishing the reliability of simulated metabolism continues to be pivotal in accepting predictions of both fate and toxicological endpoints, especially when metabolic activation of a parent chemical is deemed crucial. A quintessential way of estimating the reliability of simulated metabolism is by comparing a simulated metabolic map with an appropriate documented metabolic map. The approach is constructed on two core parts - experimental and theoretical corroboration. Specifically, the three-layer algorithm is used to support experimentally the adequacy of the simulated maps. The first layer defines similarity boundaries between the parent chemical or metabolite starting the sequence, the root of the simulated series of biotransformations, and the corresponding initial structure of the analogue from the database with documented maps. Different criteria (e.g., the commonality between organic functional groups) are used for this rationale. The second layer delineates the metabolic transformation sequences applied to the target chemical or the initial metabolite of the transformation sequence. The last layer establishes the similarity between the final transformation product in the simulated and documented sequences. To support the adequacy of the simulated molecular transformations, a library of theoretical knowledge is used, providing mechanistic justification on applied transformations. The results of applications of the above procedure are shown using two examples.