AMES Mutagenicity
Endpoint
The in vitro Ames test identifies mutagenicity as a result of interactions of chemicals with DNA. Different strains of Salmonella typhimurium are specially constructed to detect either frameshift (e.g. strains TA1537 and TA1538) or point (e.g. strain TA1531) mutations [1]. All Salmonella strains carry some type of defective (mutant) gene that prevents them from synthesizing the amino acid histidine. In the presence of mutagenic chemicals, the defective gene may be mutated back to the functional state, allowing the bacterium to grow.
Data
Collected are 4,190 chemicals from different sources (NTP database, NIHS Japan, ECVAM database, other literature sources) with experimental Ames data separated in three categories: 1,018 positive chemicals as parents, 470 chemicals positive after S9 metabolic activation (negative as parents), and 2,702 totally negative chemicals. These three classes of chemicals were considered as biologically dissimilar in the modeling process; i.e., chemicals being positive/negative as parents are distinguished from chemicals, which are positive/negative after metabolic activation. These subsets were subsequently modeled separately by the TIMES structural alerts approach. In this respect, there are two Ames models: with and without metabolic activation included in the TIMES system.
Alerts in Ames models
The toxicodynamic component of the Ames models, i.e., alerts (functionalities reacting with DNA) have been developed by chemicals which are positive as parents (one should be reminded that chemicals which are positive only after metabolic activation are negative as parents) [2]. Alerts associated with DNA interactions consist of boundaries, which provide the minimum structural requirements for interacting with DNA. Usually, additional structural and parametric boundaries are required for completing definition of alerts.
A subset of the training set is associated with each alert. In fact, these are chemicals used to define alerts boundaries. Cardinality of these local training sets depends on the number of substances captured by the alert boundaries. Only those alerts are included in the model for which a clear interpretation of the molecular interaction mechanism could be provided. Currently, the total number of alertinggroups included in the TIMES Ames models is 91 [3].
Ames model without accounting for metabolic activation (TIMES_Ames (-S9))
The training set of the Ames model (-S9) comprises chemicals which are positive (1,018) and negative (3,172) as parents. In this respect, the model predicts DNA-damaging effect of parent chemicals, only. Positive predictions are obtained when the complete set of structural/parametric boundaries of the alert(s) are met in the parent chemicals.
According to the performance of TIMES_Ames model (-S9) when applied on the training set chemicals, correct predictions are provided for:
- 855 out of 1,018 observed positive parent (Sensitivity = 84 %)
- 3,013 out of 3,172 observed negative parents (Specificity = 95%)
Ames model accounting for metabolic activation (TIMES_Ames (+S9))
The training set of the Ames model (+S9) comprises substances which are positive (470) and negative (2,702) after metabolic activation. Since both Ames models (-S9 and +S9) share the same alerts, the Ames model (+S9) predicts DNA-damaging effect of parent chemicals and their metabolic products, obtained after S9 metabolic activation. The toxicokinetic component of the model consists of a metabolic simulator which is trained to reproduce documented maps for mammalian liver metabolism of 261 chemicals. The number of generated metabolites is manageable which allows combining in a single modeling platform metabolic simulation of chemicals and their interaction with target macromolecules. The S9 metabolic simulator has its own training set which includes 542 metabolic reactions:
- Phase I transformations, such as aliphatic C-oxidation, aromatic C-hydroxylation, oxidative N- and O-dealkylation, epoxidation, ester and amide hydrolysis, carbonyl group reduction, nitro group reduction, N-hydroxylation, etc.
- Phase II transformations, such as glucuronidation, sulfation, glutathione conjugation, N-acetylation, etc.
A characteristic feature of the in vitro metabolic simulator is the significant prevalence of Phase I reactions (85-90%) over Phase II reactions, which reflects the specificity of the in vitro experimental systems with respect to their metabolic capacity [4]. The metabolic simulator cannot estimate the reaction rates (e.g. kinetic constants, half-lives, etc.) but is able to provide tentative information for their probability. The logic of the Ames mutagenicity model (+S9) is illustrated in Figure 1:
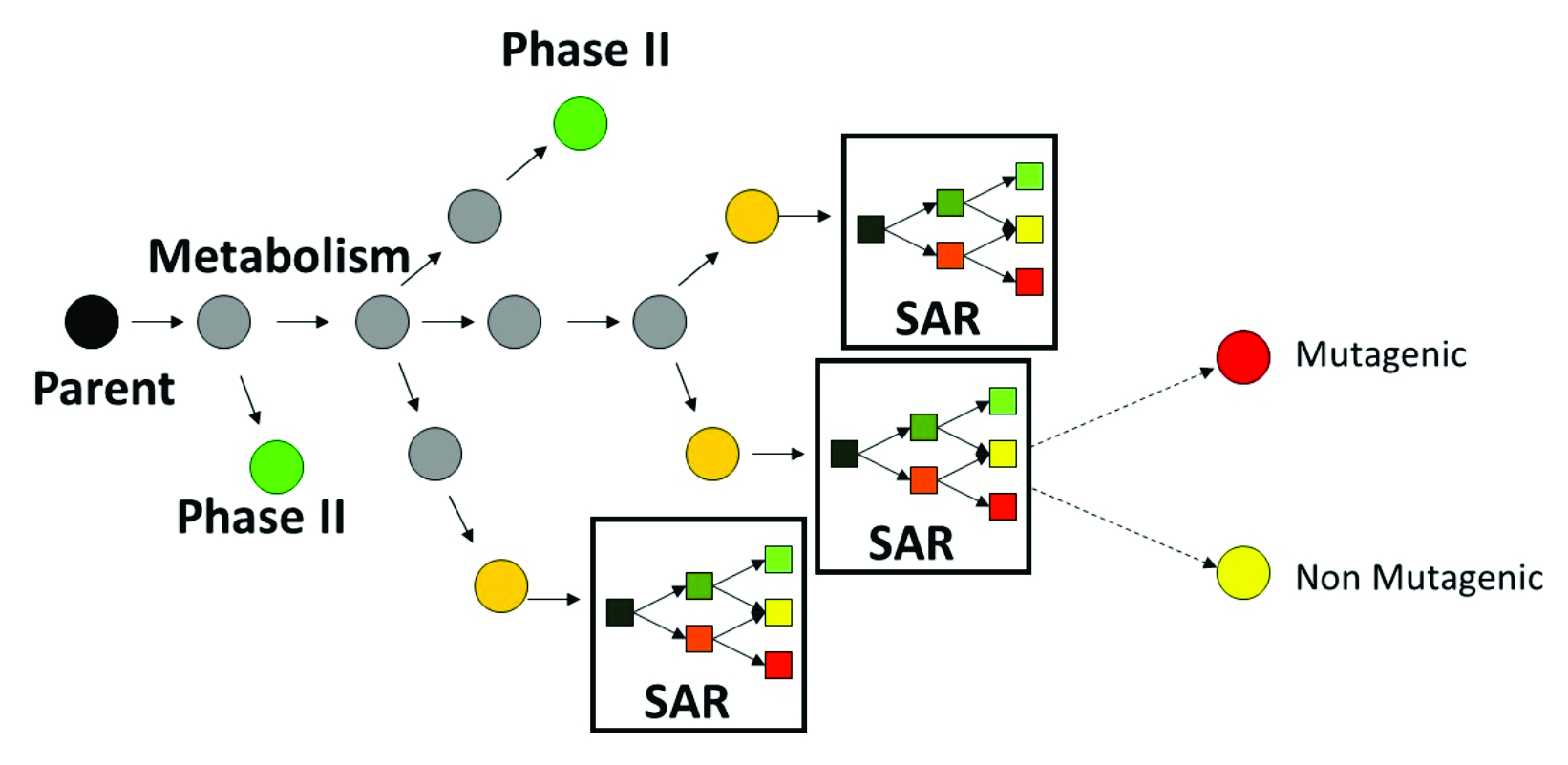
Figure 1. Illustration of the integral Ames mutagenicity prediction based on metabolic simulator and defined structure-activity relationships (SARs).
Parent chemicals and each of the generated metabolites are submitted to a battery of SARs (alerts) to screen for a general Ames effect and mutagenicity mechanisms. Thus, chemicals are predicted to be mutagenic as parents only, parents and metabolites, and metabolites only.
According to the performance of TIMES_Ames model (+S9) when applied on the training set chemicals, correct predictions are provided for:
- 373 out of 470 observed positive after metabolic activation (Sensitivity = 79 %)
- 2,196 out of 2,702 observed negative after metabolic activation (Specificity = 81 %)
Domain
Each of the TIMES Ames models (-S9 and +S9) has its own model applicability domain, which supports the Ames predictions [5]. The applicability domains consist of three sub-domain layers: general parametric requirements, structural features and alert(s) reliability. Two chemical subsets are used for deriving the model domains. The first subset includes the training set chemicals which are correctly predicted by the models, whereas the second subset comprises training set chemicals which are incorrectly predicted by the models.
The correct chemical subset is used for defining the general parametric requirements. Extracted are specific ranges of the molecular weight (MW) and the 1-octanol/water partition coefficient (log KOW):
• Molecular weight MW (in Da) ϵ [31; 1,614],
• log KOW ϵ [-18, 22].
The atom-centered fragments extracted from the correct subset of chemicals are used to define the structural domain. Briefly, the structural domain is assessed based on atom-centered fragments, extracted from correctly and incorrectly predicted (i.e., false positives and false negatives) substances from the model training sets by accounting for the atom type, hybridization and attached H-atoms of the central atom and its first neighbours. If the neighbour is a heteroatom then the diameter of the fragment is increased up to three consecutive heteroatoms or to the first carbon atoms in sp3 hybridization. In order to assess if a new chemical belongs to the structural domain, the system partitions the chemical to atom-centered fragments, which are then matched to the fragments extracted from the correct and incorrect chemical subsets. The new chemical is estimated to belong to the structural domain only when its atom-centered fragments are found in the list of correct fragments.
The third level of the domain accounts for reliability of alerts. Currently, information for alerts reliability is provided in the model reports.
External validation of the TIMES Ames (+S9) model
A large number of chemicals (12,000) with highly reliable Ames data have been used in a screening exercise of NIHS Japan to help increasing the predictive power of the major QSAR models for predicting Ames mutagenicity (http://www.nihs.go.jp/dgm/amesqsar.html). This exercise is scheduled for accomplishing in three Phases by distributing approximately 4,000 chemicals for screening in each Phase. So far, Phase I and II trails have been completed and the results were presented in the 17th International Conference on QSAR in Environmental and Health Sciences in Miami Beach, Florida USA in June of 2016.
In the Phase I and II trials, Ames data of totally 7,731 substances were delivered to vendors for screening by their Ames model. The Ames assays were conducted according to the OECD TG471 and Industrial Safety and Health Act in Japan under GLP-compliant conditions. Based on their Ames data, investigated chemicals are classified into three categories:
- Strongly positive mutagens (Class A) - generally induce more than 1,000 revertants/mg in at least one Ames strains in the presence or absence of rat S9,
- Positive mutagens (Class B) - reproducibly induce revertant colonies with dose-dependent manner, different from Class A mutagens,
- Negative Ames substances (Class C).
As a result of the screening, when accounting for the model domain, the TIMES Ames model (+S9) shows balanced predictions in terms of sensitivity of 80% (for Class A chemicals) and specificity of 91%. This result is acceptable given the interlaboratory reproducibility of Ames data which is 85% [6]. The Ames model (-S9) has not been evaluated in this screening exercise, because the experimental Ames data are provided without information whether they are obtained with or without S9 metabolic activation. Due to the lack of information for the presence or absence of S9 metabolic activation (critically important for TIMES models), the screened chemicals cannot be used for modifying the TIMES Ames models.
Reporting
The predictions of in vitro Ames mutagenicity models could be reported as:
- A tab delimited text file providing the following information for chemical: identity (CAS number, Name, SMILES), observed Ames mutagenicity, predicted Ames mutagenicity, information for the alert(s) responsible for DNA interaction, alert reliability and performance, applicability domain, etc.
- QSAR Prediction Reporting Format (QPRF) is available, generating a report for one chemical or group of chemicals.
The TIMES Ames (+S9) model provides information for:
- Simulated metabolic map,
- Positive parents and/or metabolites,
- Mechanisms of DNA damage,
- Local training set of alerts,
- Performance of alerts,
- Different types of reporting
- …..
Decision support character of TIMES_Ames models
Users of the TIMES model may assess reliability of the predictions by evaluating the amount of mechanistic justification supporting the ultimate prediction. In this respect, the modeling results could be considered as decision supporting rather than decision making outcome.
References
1. Ames, B., McCann, J., Yamasaki, E., 1975. Methods for Detecting Carcinogens and Mutagens with the Salmonella/Mammalian-Microsome Mutagenicity Test. Mutation Res., 31, pp. 347-364.
2. Serafimova, R., Todorov, M., Pavlov, T., Kotov, S., Jacob, E., Aptula, A., Mekenyan, O. 2007. Identification of the structural requirements for mutagenicity by incorporating molecular flexibility and metabolic activation of chemicals. II. General Ames mutagenicity model. Chem. Res. Toxicol, 20, pp. 662−676.
3. Petkov, P.I., Schultz, T.W., Donner, E.M., Honma, M., Morita, T., Hamada, Sh., Wakata, A., Mishima, M., Maniwa, J., Todorov, M., Kaloyanova, E., Kotov, S., Mekanyan, O. 2016. Integrated approach to testing and assessment for predicting rodent genotoxic carcinogenicity,J. Appl. Toxicol.36(12): 1536-1550.
4. Mekenyan, O., Dimitrov, S., Pavlov, T., Dimitrova, G., Todorov, M., Petkov, P., Kotov, S. 2012. Simulation of chemical metabolism for fate and hazard assessment. V. Mammalian hazard assessment, SAR and QSAR in Environmental Research, 23:5-6, 553-606.
5. Dimitrov, S., Dimitrova, G., Pavlov, T., Dimitrova, N., Patlevisz, G., Niemela, J., and Mekenyan, O. 2005. A stepwise approach for defining the applicability domain of SAR and QSAR models, J. Chem. Inf. Model. Vol. 45 (2005), pp. 839-849.
6. Piegorsch, W.W., Zeiger, E. 1991. Measuring intra-assay agreement for the Ames Salmonella assay. Lecture notes in Medical Informatics; Springer-Verlag: Heidelberg: 35-41.

